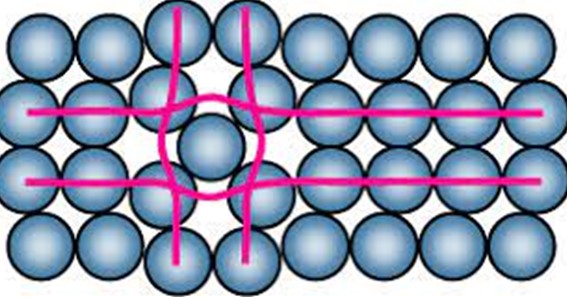
Are you curious to know what is point defect? You have come to the right place as I am going to tell you everything about point defect in a very simple explanation. Without further discussion let’s begin to know what is point defect?
In the fascinating world of materials science, even seemingly perfect crystalline structures are not without flaws. These imperfections, known as point defects, play a significant role in determining the properties and behavior of materials. In this blog post, we will delve into the concept of point defects, their types, and their impact on the characteristics of materials.
What Is Point Defect?
Point defects are localized imperfections that occur at the atomic scale within a crystalline material. They result from missing atoms, extra atoms, or atoms occupying incorrect lattice sites within the crystal lattice. These defects can be either inherent in the material’s structure or introduced during manufacturing or processing.
Types Of Point Defects
- Vacancies: Vacancies are the most common type of point defect. They occur when an atom is missing from its lattice site, leaving behind an empty space. Vacancies can affect the material’s density, mechanical properties, and diffusion characteristics.
- Interstitials: Interstitials are additional atoms or ions that occupy the spaces between lattice sites. They can disrupt the regular arrangement of atoms and alter the material’s mechanical properties, electrical conductivity, and diffusion behavior.
- Substitutional Defects: Substitutional defects occur when an atom is replaced by a different atom of a similar size within the crystal lattice. This type of defect can affect the material’s properties, such as its electronic structure, thermal conductivity, and chemical reactivity.
- Frenkel Defects: Frenkel defects involve the dislocation of an atom from its lattice site to an interstitial position within the crystal structure. This defect is common in materials with a large size difference between atoms, and it can affect the material’s electrical conductivity and diffusion properties.
Effects Of Point Defects
Point defects significantly influence the properties and behavior of materials. Some effects of point defects include:
- Mechanical Properties: Point defects can affect a material’s mechanical properties, such as strength, ductility, and hardness. Vacancies and interstitials can create local stress concentrations, leading to changes in material deformation and fracture behavior.
- Electrical Properties: Point defects can alter a material’s electrical conductivity, resistivity, and band structure. Substitutional defects and interstitials can introduce impurity levels within the band gap, affecting the material’s electronic properties and conductivity.
- Diffusion and Migration: Point defects play a crucial role in the diffusion and migration of atoms within materials. Vacancies and interstitials provide diffusion paths for atoms to move through the crystal lattice, influencing processes such as atomic transport and solid-state reactions.
- Optical Properties: Point defects can impact the optical properties of materials. The presence of certain defects can introduce energy levels within the band gap, affecting the absorption, emission, and scattering of light.
Applications And Control Of Point Defects
Understanding and controlling point defects is of great importance in various fields:
- Materials Engineering: Manipulating point defects allows engineers to tailor the properties of materials for specific applications. For example, doping semiconductors with specific impurities creates intentional point defects that enhance the material’s conductivity and electronic properties.
- Semiconductor Devices: Point defects play a critical role in the performance of semiconductor devices. The controlled introduction of dopants and intentional defects allows the design of transistors, diodes, and other electronic components with desired characteristics.
- Energy Storage: Point defects can influence the performance of energy storage materials, such as batteries and fuel cells. Optimizing the distribution and density of defects can improve the efficiency, stability, and lifespan of these devices.
- Catalysis: Point defects can significantly impact the catalytic activity of materials. By engineering and controlling the type and concentration of defects, catalysts can be optimized for various chemical reactions and processes.
Conclusion
Point defects, the imperfections present at the atomic scale within materials, play a crucial role in shaping the properties and behavior of crystalline materials. Understanding the different types of point defects and their effects is vital for materials scientists and engineers seeking to optimize the performance of materials for various applications. By manipulating and controlling point defects, it becomes possible to design materials with tailored properties, leading to advancements in fields such as electronics, energy storage, catalysis, and more. Point defects remind us that imperfections at the atomic level can have profound consequences in the macroscopic world.
FAQ
What Is The Point Defect?
Point defects are lattice defects of zero dimensionality, i.e., they do not possess lattice structure in any dimension. Typical point defects are impurity atoms in a pure metal, vacancies and self-interstitials.
What Is Point Defect With Example?
Point defects are accounted for when the crystallization process occurs at a very fast rate. These defects mainly happen due to deviation in the arrangement of constituting particles. In a crystalline solid, when the ideal arrangement of solids is distorted around a point/ atom it is called a point defect.
What Are Point Defects In Crystal?
Point defects are, in principle, isolated mistakes at single atom positions in a crystal. In a pure monatomic crystal, these can consist of an atom missing from a normally occupied position, a vacancy, or an atom in a position not normally occupied in the crystal, a self-interstitial.
What Are Point Defects And Line Defects Class 12?
A point defect is any defect that involves only a single particle (a lattice point) or sometimes a very small set of points. A line defect is restricted to a row of lattice points, and a plane defect involves an entire plane of lattice points in a crystal.
I Have Covered All The Following Queries And Topics In The Above Article
What Is Point Defect Class 12
What Is Point Defect In Chemistry
What Is A Point Defect
What Is Crystal Defect And Point Defects
What Is Point Defect In Crystal
Types Of Point Defect
Point Defect Example
What Is Point Defect And Describe Schottky Defect
Vacancy Defect
Stoichiometric Defect Class 12
What Is Stoichiometric Defect
Point Defects In Solids
What Is Point Defect
What is the difference between a point defect and a line defect
What are point defects?